From Prototype to Production: Allstar Magnetics’ CustomComponents Drive Innovation in Medical Devices
Client: A leading medical device manufacturer specializing in equipment for arthroscopic surgery.
Industry: Medical Devices
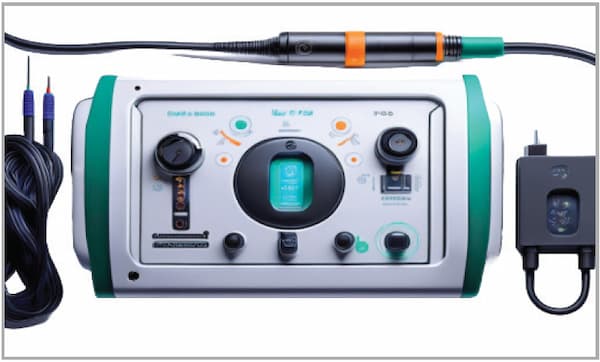
Project Overview: A medical device manufacturer approached Allstar Magnetics with a need for custom wound components to power their nextgeneration radio frequency (RF) generator, used in arthroscopic surgery. The RF generator was designed to use energy for precise surgical tasks, and they required custom transformers and inductors to meet both regulatory and functional demands. The challenge was to develop components that provided reliable performance, power isolation for patient safety, and compliance with FDA requirements, while maintaining efficient manufacturability.
Allstar’s Approach: Allstar Magnetics engaged in a collaborative partnership with the client, acting as an extension of their development team. The project involved developing ten different custom wound components, including transformers for power regulation and critical patient isolation.
Client Testimonial: While engineers rarely express feedback explicitly, the ongoing demand for Allstar’s components after 30 years speaks volumes about the effectiveness of the collaboration. The medical device manufacturer continues to rely on Allstar for the same custom wound components, even as the product has evolved to meet modern medical standards.
Key aspects of the project included:
- Consultative Design Support: The client provided basic specifications, and Allstar helped shape the technical design, material selection, and manufacturability to ensure that the components would meet electrical and regulatory requirements. Allstar proposed configurations, produced prototypes, and collaborated with the client through multiple iterations to finalize designs.
- Patient Isolation Transformer: A crucial component was the patient isolation transformer, which provided necessary separation between the device’s power source and the patient to meet FDA safety standards. This transformer played a pivotal role in preventing electrical risks during surgery.
- Prototyping and Testing: Allstar provided multiple rounds of prototypes for the client’s testing and proof of concept. Throughout the process, adjustments were made to ensure that the components performed optimally in the medical device’s RF generator.
- Scalable Production: After successful testing and design finalization, Allstar scaled the production of the components, leveraging both in-house and offshore manufacturing capabilities. The design allowed for easy scalability to meet the growing demand for the medical device.
Results:
- Successful Product Launch: The RF generator launched successfully, and the device gained traction in the medical community, particularly in arthroscopic surgery for procedures like rotator cuff repair and tissue ablation. The components designed by Allstar have remained integral to the product’s long-standing success.
- Compliance with FDA Regulations: The power isolation transformers provided by Allstar ensured full compliance with FDA regulations, safeguarding patients from electrical risks during surgical procedures.
- Long-Term Partnership: The success of the RF generator contributed to a lasting relationship between Allstar and the client. Even as the original manufacturer was acquired multiple times, Allstar continued to supply the components for ongoing product iterations, making the collaboration a cornerstone of the device’s market longevity.
Conclusion: Allstar Magnetics’ expertise in custom wound components and collaborative design support allowed the medical device manufacturer to bring an innovative RF generator to market. The partnership enabled seamless scaling, compliance with stringent regulations, and continuous product improvement, contributing to the device’s success in the medical industry for over three decades.